ABOUT US
OUR STORY
MOBERG ANALYTICS, EST. 2020
Our Mission
Leverage AI, machine learning, and next-gen analytics to enable precision care for acute brain-injured patients.
Our Purpose
Balance product development with community education on latest advancements in medical technology.
Our Way
Combine expertise in neuromonitoring, cloud technology, and AI to harness the power of multimodal data.

The Neurotrac ®, developed in part by Dick Moberg, revolutionizes the field with the first use of quantitative EEG, and is the first commercial instrument to trend Spectral Edge Frequency. It replaces traditional manual EEG interpretation with quantifiable, numerical data. As the first microprocessor-based instrument for performing EEG and somatosensory evoked potentials, it quickly gains global recognition for its use in surgery and critical care.

Dick Moberg founds Moberg Medical as a spin-off from his previous company, Interspec, where the Neurotrac I was invented. Moberg Medical is established with the intent to focus on advancing neuromonitoring devices.

The Neuro Science Monitor, now the oldest newsletter on neuromonitoring, publishes its first edition in the Summer of 1990. With a playful tone and engaging content, it aims to inform and entertain readers while promoting multimodal neuromonitoring advancements and upholding unbiased reporting in neuroscience innovation. Today, it remains true to these principles.
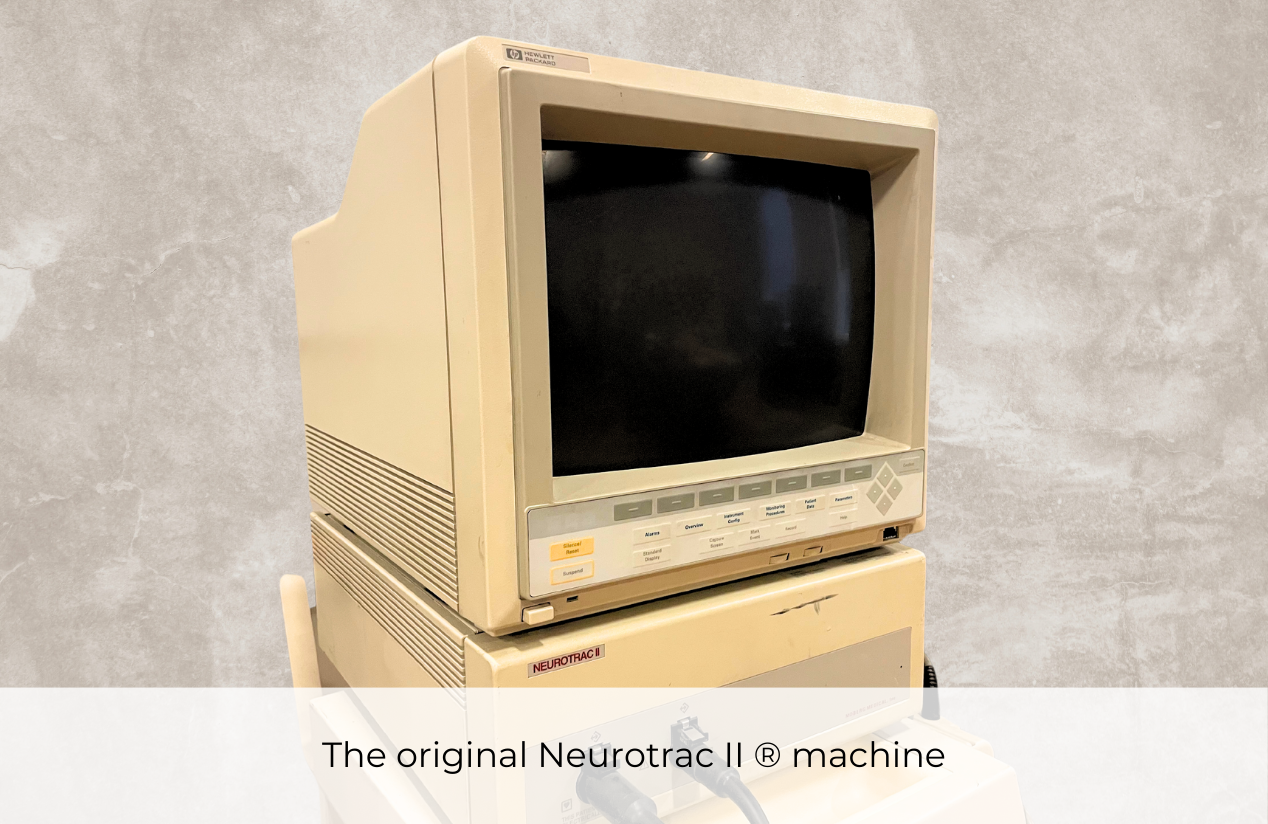
The Neurotrac II ®, the flagship product of Moberg Medical, builds on its predecessor by introducing pioneering features such as multimodal brain function monitoring that combines EEG, evoked potentials, and transcranial Doppler ultrasound. It is among the first to introduce continuous EEG monitoring in critical care, and is recognized as the easiest EEG monitor to use by anesthesia and critical care personnel.

The Grateful Head ®, the first EEG simulator designed for educational purposes, is developed at Moberg Medical. It revolutionizes EEG education by providing a practical simulation tool for training, demonstrations, and troubleshooting, as well as generating physiological waveforms, including continuous EEGs and evoked potentials.

Building on previous successes, Moberg Research – following the sale of Moberg Medical to Telefactor – seeks to innovate in critical care monitoring by leveraging expertise gained in surgical monitoring settings.

Recognizing the need for a dedicated patient monitor for brain activity and integrated neuromonitoring in critical care, the CNS Monitor is developed at Moberg Research to consolidate data from various brain monitors into a single device for nurses' ease. Offering high-resolution data collection and correlation visualization, it becomes the standard system for traumatic brain injury (TBI) trials and the preferred choice for monitoring spreading depolarizations.

BrainBall ®, a game co-invented by Dick Moberg, makes its debut. Players utilize relaxation techniques to control a ball's movement on the BrainBall table. Commercialized by Moberg Research, it is showcased widely across the U.S. and Europe and emerges as a tool for education on multimodal monitoring.
Amidst a historic recession in November of 2008, the CNS Monitor is cleared by the FDA, making it the first FDA-cleared device to integrate EEG with data from other monitors. This marks the official start of the field of multimodal neuromonitoring. The CNS Monitor persists in research, development, and diversified business units, leading Moberg Research to become a profitable and rapidly growing business as the market rebounds.

Moberg Research undergoes a rebranding, becoming Moberg ICU Solutions. Positioned at the forefront of integrated neuromonitoring, the company pioneers core technology for bedside patient management. Securing funding from NIH and the DoD to advance its innovations, Moberg ICU Solutions successfully sells the CNS Monitor to major hospitals, with some adopting the product for every bed in their neuro ICU.

In 2014, the CNS Monitor is deployed as the primary data collection system in both the TRACK-TBI and CENTER-TBI studies, emphasizing its crucial contribution to the progression of research and treatment methodologies for TBI.
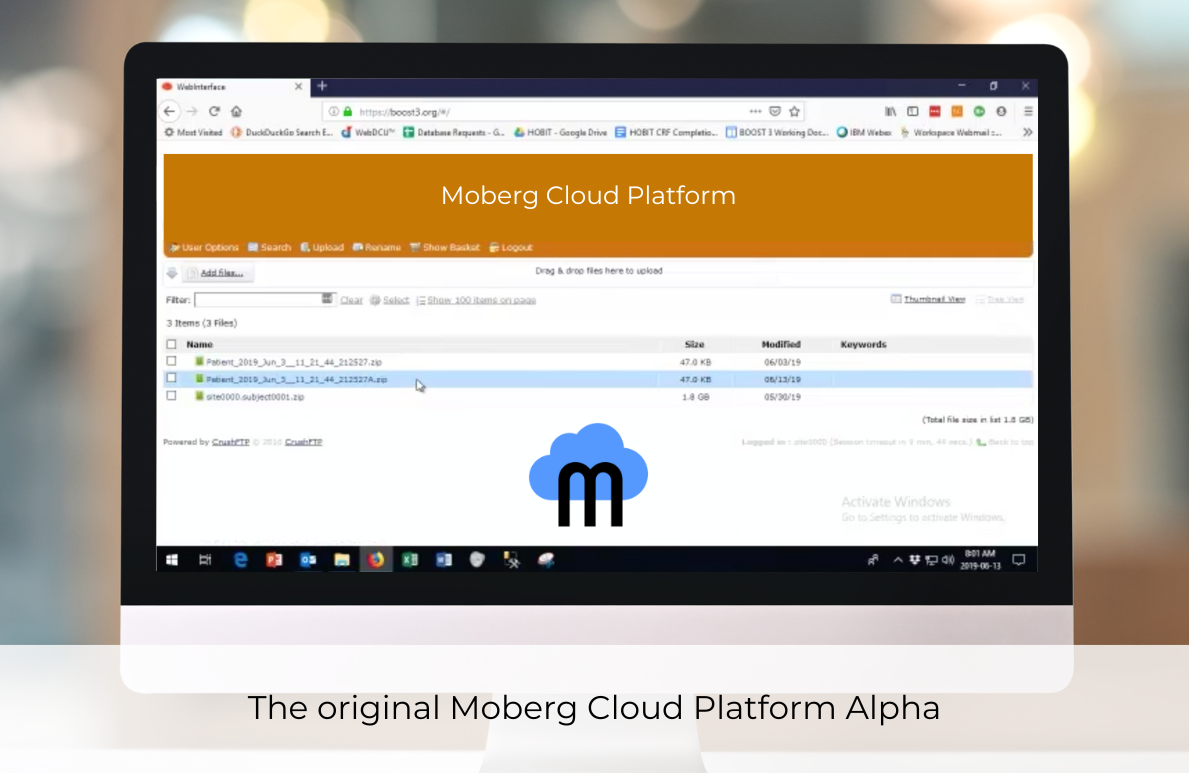
Although the CNS Monitor solved the data aggregation problem, it simultaneously created a Big Data problem in neuromonitoring. To tackle this, Moberg ICU Solutions launches the Moberg Cloud Platform Alpha (formerly Moberg CONNECT) in 2019. The platform addresses the increasing demand for managing extensive data generated by multimodal monitoring in severe brain injury cases.

In 2019, Moberg ICU Solutions is acquired by ArchiMed, a prominent European private equity group, through its portfolio company Micromed. (Later, in 2023, Micromed is acquired by Natus, the global leader in neurophysiology, greatly benefitting the Moberg CNS Monitor).

Amidst the pandemic, Moberg Analytics is founded with a $4.5 million grant from the U.S. Army to create a "New Medical Record for the Brain," focusing on data aggregation for personalized patient care in neurocritical settings. Building upon the expertise and innovations of its predecessor, the venture aims to advance data analytics solutions for neurocritical care, particularly by maximizing the value of data collected by the CNS Monitor from the injured brain.

The Moberg Cloud Platform Alpha, now managed by Moberg Analytics, is deployed in major brain injury clinical trials including BOOST-3, a study comparing the effectiveness and safety of ICU treatment strategies for TBI using either both intracranial pressure (ICP) and brain tissue oxygen (PbtO2) levels, or ICP alone.

Moberg Analytics receives $3 million from MTEC to develop the TBI Navigator: portable multimodal neuromonitoring for combat scenarios. Collaborating with companies and clinical teams, the project adapts existing technologies for managing TBI in soldiers, supporting Moberg Analytics’ mission to enhance precision care in diverse environments.

A year after its founding, Moberg Analytics transitions from remote work during the pandemic to an in-person office in Midtown, Philadelphia. The company soon expands its workforce, tripling office space and boosting capabilities in research, development, training, and production.

The Moberg Cloud Platform Beta is selected for the PRECICECAP Clinical Trial, aiding in the study’s mission to apply machine learning to multimodal data from cardiac arrest patients to identify biomarkers for optimal hypothermia duration and outcomes. PRECICECAP directs its NIH funding to Moberg Analytics to focus on AI and machine learning readiness.

The Moberg Cloud Platform Beta is selected for both the AUTO-BOOST and ELECTRO-BOOST Clinical Trials. AUTO-BOOST examines machine learning analytics of heart rate variability using waveforms collected by the Moberg CNS Monitor as predictors of parameters believed to affect cerebrovascular reactivity and outcomes. ELECTRO-BOOST studies the relationship between PbtO2, EEG abnormalities, clinical outcomes, and treatments to identify dynamic EEG biomarkers of secondary injury.

In early January, Moberg Analytics hosts a successful two-day workshop at company headquarters in Philadelphia with ICM+, focusing on multimodal neuromonitoring technologies developed by Dick Moberg and Peter Smielewski. The workshop provides hands-on sessions, technical talks, and discussions on the future of neurocritical care, setting the stage for advancements in the field.
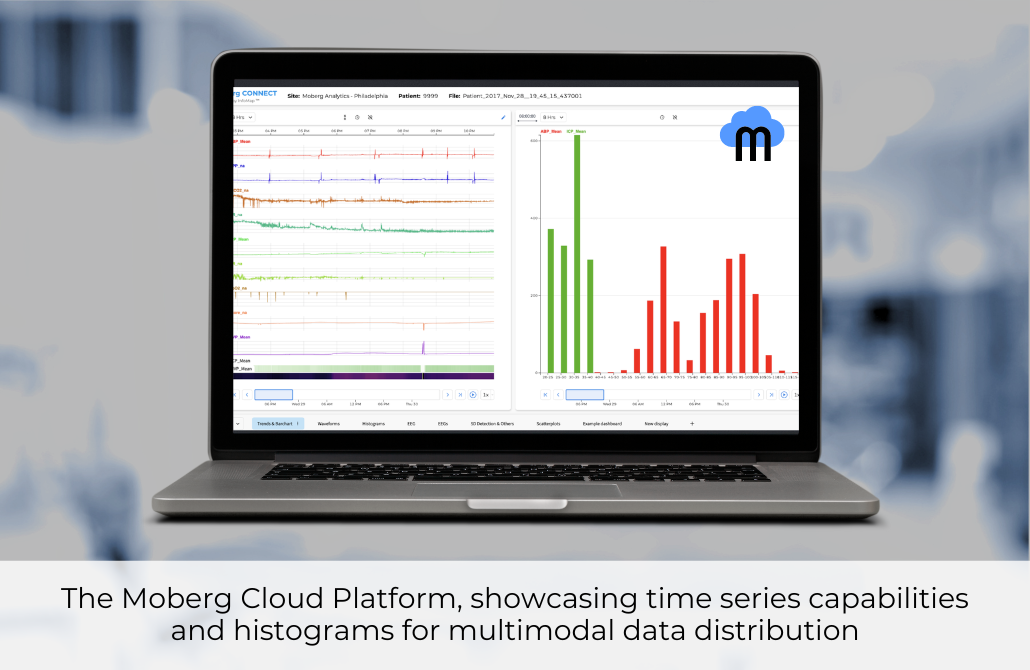
The production version of the Moberg Cloud Platform is released, offering a comprehensive neurocritical care data management toolbox. This software facilitates uploading, visualizing, annotating, and analyzing high-resolution data, as well as summarizing de-identified bedside data into multimodal reports for multidisciplinary research teams. Earlier versions of the software have been instrumental in major TBI clinical trials.

Moberg Analytics is selected by MTEC and TATRC to develop the Army's AutoDoc (Autonomous Documentation) Sensor, a system that will passively collect real-time data to automate documentation, allowing medics to focus on care while improving logistics, triage, and evacuation for better health and survival outcomes.

The TBI Navigator App is launched in September of 2024, and is made available on the Apple App Store and Google Play. It is a groundbreaking mobile app offering clinical decision support for the assessment and management of acute brain injury in prehospital and hospital settings.
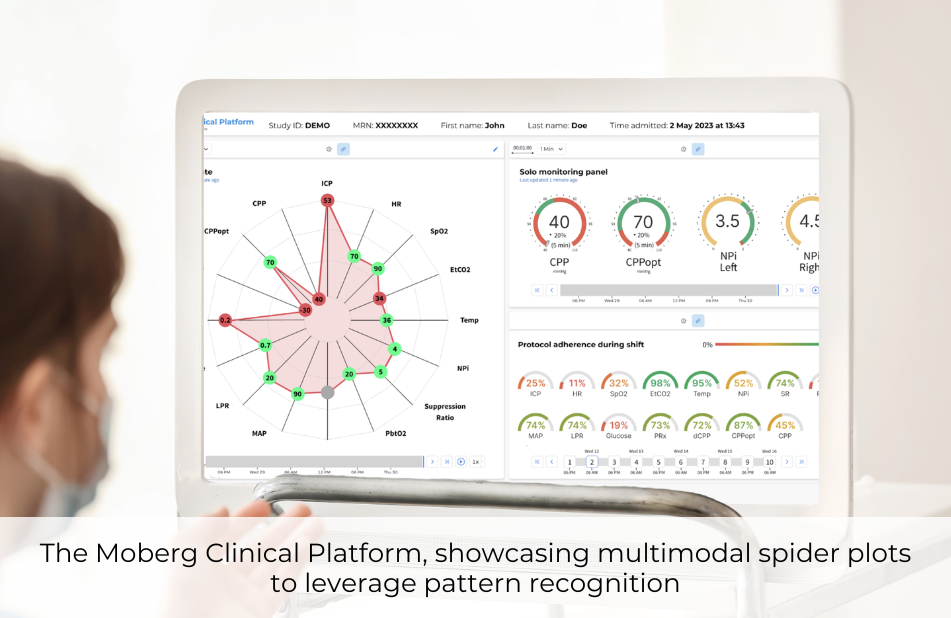
Moberg Analytics will launch the Moberg Clinical Platform, further advancing the field of neurocritical care by leveraging data acquired at bedside to inform individualized care and quality improvement. Its comprehensive data acquisition, integrated multimodal displays, and next-gen analytics make it a revolutionary clinical tool.

